49 Mall Drive was purchased in 2015 by Ropack Pharma Solutions (RPS). RPS has made the strategic decision to market and sell its solid oral dosage development and manufacturing facility located at 49 Mall Drive, Commack, New York. This
state-of-the-art 103,530 sq-ft. facility is located on Long Island, less than 50 miles from New York City and includes process development and commercial manufacturing suites, as well as full fit-out analytical laboratories.
This facility has fully validated HVAC’s, USP water system, compressed air, laboratories, warehouses and manufacturing clean rooms from a cGMP standpoint. Complete equipment train and laboratory devices allow the capacity to perform a wide range of cGMP activities from formulation development to mid-sized commercial productions.
HIGHLIGHTED FEATURES:
• cGMP compliant facility
• 2 billion tablets/year production capacity
• State-of-the-art solid dosage development and manufacturing suites and equipment , including 16 cGMP manufacturing suites
• Over $50M in capital investments in the past 10 years
• Excellent regulatory history
• Temperature-and humidity-controlled production suites
• DEA Schedule II-V license
• Solid utility infrastructure, with an actual completed commissioning
• Up to 40,000 sq ft of warehouse expansion possible on available land
• All actual and current IDA and Empire State grants are transferable to new owners
• Immediate, easy freeway access (I-495)
KEY DEPARTMENTS:
• Formulation
• Process Development
• Clinical Manufacturing
• Commercial Manufacturing
• Analytical Development
• Pre-Formulation
• PR&D Project Management
• Quality Assurance
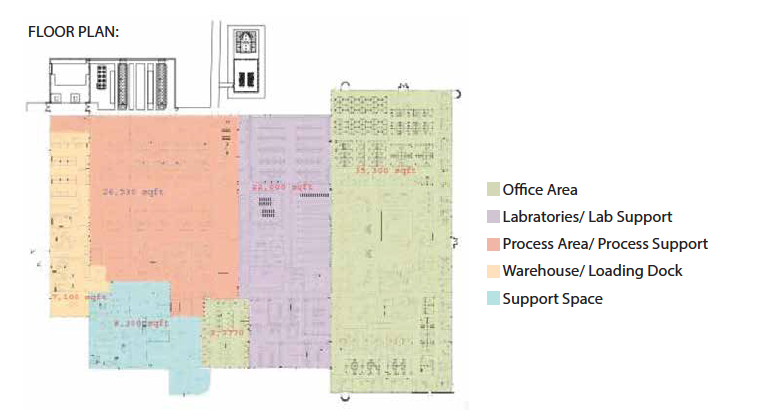
BUILDING UTILIZATION (square-feet)
GMP Warehouse: 4,861
GMP Manufacturing: 22,075
Distribution: 1,474
Record Archives: 212
Office: 22,677
Cafeteria: 3,220
Conference/Training Rooms: Laboratory: 16,240
Corridors/Restrooms/Other: Mechanical: 6,645
Total: 103,358
SPECIFICATIONS:
LAND: 10.75 acres
LOADING: 2 loading docks
HEIGHT: 22’6” (warehouse)
ELECTRIC: Service 1: 1600 Amp, 480/277 volt
Service 2: 1600 Amp, 480/277 volt
Service 3: 2000 Amp, 480/277 volt HEATING: Gas
UPS: 1 – Powerware Plus 225 (225 KVA)
2 – Powerware Plus 40 (40 KVA)
GENERATOR: Kohler Diesel Generator (300 KW)







SITE LOCATION:
49 Mall Drive Commack, NY 11725
HEAD OFFICE:
10801 Mirabeau Street, Montreal, QC
Canada H1J 1T7
Tel: 514.353.7000;2209
RPS has operated in this facility with the belief that advanced technology brings advanced results. We have applied this principle in keeping the labs pristine and production suites equipped and fully commissioned utility systems, with technology from the most respected names in the industry to meet cGMP requirements.
Interested parties should contact Yves Massicotte, the President and CEO of RPS, for more information regarding an acquisition of this Commack, New York-based solid dosage development and manufacturing facility.
For further information, please contact:
Yves Massicotte, President and CEO
Josee Leblanc, Assistant to the President & CEO
josee.leblanc@ropack.com
1-888-353-7090 ext: 2271